Gary Horgan (CMSE Consultancy Manager at the Chris Mee Group) and his team are outlining the path for companies to ensure they are compliant with Part 8 “Explosive Atmospheres at Places of Work” of the Health, Safety & Welfare at Work (General Applications) Regulation 2007 in a series of focussed blogs.
- Blog 1, Explosion Accidents 2020
- Blog 2, Preparing for an Explosion Protection Document
- Blog 3, Summary of Legal Requirements
- Blog 4, What Are Explosions?
- Blog 5, Characteristics of Flammable Liquids, Gases and Vapours
- Blog 6, Hazardous Area Classification – Dust
- Blog 7, What are the hazards of liquid nitrogen and why should we be concerned?

Liquid nitrogen (N2) is a form of nitrogen gas which is inert, colorless, non-irritating and odorless. At extremely low temperatures (-195oC) it forms a liquid and is referred to as a cryogenic liquid. An important consideration of cryogenic liquids is their ability to produce large amounts of gas when vaporized.
Liquid nitrogen has many industrial uses, including:
- Freezing and transport of food products
- Preservation of biological samples
- The shielding of materials from oxygen exposure
- Cooling of materials for easier machining
For industrial use, liquid nitrogen is primarily stored and handled in cryogenic liquid cylinders and cryogenic storage tanks. However, releases of liquid nitrogen during a process can occur unexpectedly leading to severe health effect exposure to people in the workplace. The most common sources of gas release may occur through a variety of processes, such as:
- Natural release i.e. from cryogenic tanks or flasks, through evaporation from non-pressurized containers or pressure relief valve from pressurized containers.
- Release when used for routine processes. For example, processes include chilling and freezing operations or tank relief valves.
- Unintentional release due to leaks from pipework and valves.
With each above scenario consideration on the potential risk and hazards of liquid nitrogen exposure is necessary.
What are the main hazards associated with the use of Liquid Nitrogen?
Nitrogen gas is often referred to as a “silent killer” as there is no warning to humans (such as taste, smell) when exposure occurs. Therefore, it is important to understand the main hazards associated with using liquid nitrogen. Most common hazards include:
- Asphyxiation
Nitrogen gas is known as a simple asphyxiant. These gases are likely to be a danger when the concentration in inhaled air is substantial enough to cause a reduction in oxygen levels in the atmosphere. If the level of O2 in the atmosphere is reduced to below 18% this can lead to rapid breathing followed by loss of consciousness and death when levels reduce further.
Below is a summary of the effects of reduced O2 in the atmosphere:
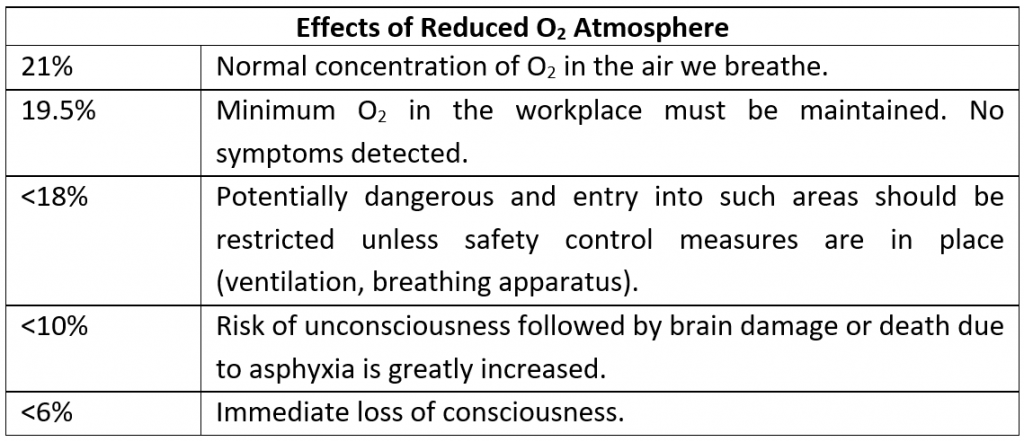
There are some factors which may increase the risk of a reduced oxygen atmosphere in the workplace, such as:
- Volume of air within the workplace. i.e. dangers of working in confined space where oxygen may be lacking due to displacement of air by another gas.
- Ventilation in the workplace (fresh air change rate)
- The volume and rate of the gas released
2. Frostbite and cold burns
Liquid nitrogen can cause significant damage and burns when in contact with the skin.
3. Explosion due to system pressurisation
When heat is applied to liquid nitrogen the pressure in the vessel or container will build up causing rapid expansion generating nitrogen gas. If liquid nitrogen is contained in a sealed or poorly vented system this will cause an explosion.
Fortunately, the reported incidents of adverse workplace exposure to N2 have remained low in Ireland. Nonetheless, it is important not to be complacent as the consequences of such incidents can be significant. In January 2021, six people unfortunately died while working at a food processing plant based in the US due to exposure to liquid nitrogen. Although the case remains under investigation, it is suspected the incident occurred due to a leak of a cryogenic freezing system where the poultry is processed.
Another significant incident of note occurred back in 2000 where a lab worker at a UK based medical research facility died from asphyxiation due to exposure to liquid nitrogen. The leak occurred in a freezer room, which caused the oxygen in the air to become depleted. Findings from the investigation concluded the cause of the exposure was due to inadequate ventilation and failure to install monitoring systems for detecting nitrogen in the room atmosphere.
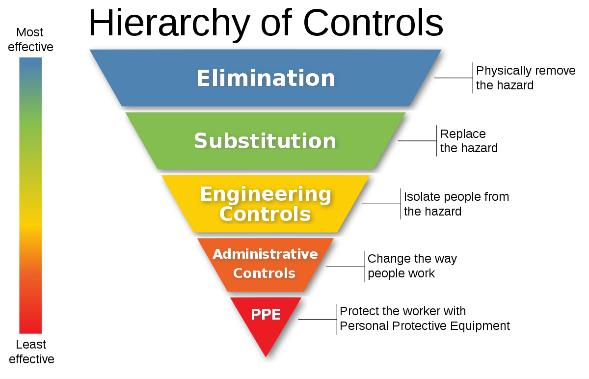
What does the employer need to do?
With any hazard in the work place the employer should always evaluate the level of risk with consideration to the Hierarchy of Controls. Asking the right questions can be beneficial in determining control measures. Can the process or substance be eliminated or substituted? Are there engineering controls to be considered i.e. isolation, ventilation? Are employees sufficiently trained i.e. work permits? When should RPE and PPE be worn?
Risk assessments, monitoring systems and emergency procedures are also essential control measures in preventing adverse N2 exposure in the workplace. Most importantly, the employer must ensure they provide adequate information, instruction and training to employees working with liquid nitrogen.
CMSE Consultancy can assist you in determining/ calculating potential hazards posed by N2 in your workplace in accordance with BCGA GUIDANCE NOTE GN11 Use of Gases in the Workplace: The management of risks associated with reduced oxygen atmospheres.
Further interesting information is available on our weekly Process Safety Blogs.
CMSE Consultancy provide a professional Health, Process, Explosion & Fire Safety Services.
If you require further information or assistance please contact us via email at info@cmse.ie, by phone at 021 497 8100 or start an instant chat with us via the chat box in the bottom right-hand corner of your screen.
