Characteristics of Flammable Liquids, Gases and Vapours
Gary Horgan and his team are outlining the path for companies to ensure they are compliant with Part 8 “Explosive Atmospheres at Places of Work” of the Health, Safety & Welfare at Work (General Applications) Regulation 2007 in a series of focussed blogs.
This is Blog number 5 in the series.
- Blog 1, Explosion Accidents 2020
- Blog 2, Preparing for an Explosion Protection Document
- Blog 3, Summary of Legal Requirements
- Blog 4, What Are Explosions?
- Blog 5, Characteristics of Flammable Liquids, Gases and Vapours
In this latest blog of our series looking at explosion safety, we will be discussing some of the key flammable characteristics of liquids, gases, and vapours.
Understanding these characteristics of the flammable materials on your site is a crucial step in the development of an Explosion Protection Document, as without this information it is impossible to understand the explosion hazards which are present.
The table below is an example of liquid, gases, and vapour characteristics that would typically be included in an Explosion Protection Document; we will look at some of these in more detail below.
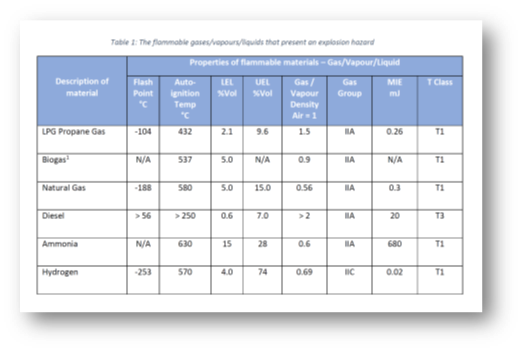
Flash Point
The flash point is the lowest temperature at which sufficient vapour is produced from a flammable liquid that ignition will occur when an ignition source is applied.
It is a critical characteristic to understand when determining the fire/explosive hazard of a material; liquids which are used at a suitable safety margin below their flash point (e.g. 10 °C) will be significantly less likely to pose an explosive hazard during normal operations.
For example, petrol has a flash point of around – 40 °C whereas diesel has a flash point of around 58 °C. In normal Irish atmospheric conditions, petrol poses a far higher fire/explosion hazard in comparison to diesel.
In fact, flash point is key to the classification of a substance’s flammability hazard according to the EU Classification, Labelling and Packaging (CLP) Regulation.
Upper and Lower Flammable/Explosive Limits
It is important to know that it is the vapour of a substance that burns. A flammable liquid must be heated to a temperature where vapour is given off (i.e. the flash point), before combustion can take place.
A flammable gas or vapour will only continue to burn in air if the mixture lies between certain limits. These limits are normally given as a percentage of the substance relative to air, and are called:
- Lower Flammable Limit (LFL) – the lowest mixture of fuel and air that will support a flame
- Upper Flammable Limit (UFL) – the highest mixture of fuel and air that will support a flame
These limits are also called the Upper and Lower Explosive Limits (UEL and LEL) – it generally agreed that these terms are interchangeable. The range between these two limits is known as the flammable range; as an example the flammable range of petrol is between 1% – 6%.
These terms are shown graphically in the sketch below:
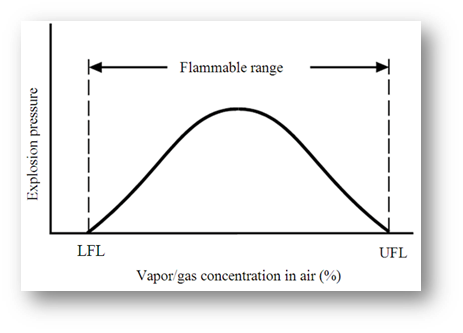
If the vapour/gas concentration is below the LFL, then the mixture is described as “too lean” and will not ignite. Similarly, if it is above the UFL, then the mixture is “too rich” and will also not ignite.
The ideal (stochiometric) mixture occurs around the mid-point of the flammable range, giving the highest burning rates and highest burning temperatures.
Auto-Ignition Temperature
The auto-ignition temperature (AIT) is the lowest temperature at which a substance will ignite spontaneously and burn without a flame or other ignition source. As an example, the AIT of petrol can be up to 280 °C, depending on grade.
It is often confused with flash point, but the key difference is that at the AIT, a flammable material can ignite without any ignition source being present.
It is therefore very important to ensure a reasonable safety margin between the operating temperature of a flammable material and its auto-ignition temperature. Specifying safe equipment with this in mind will be covered in more detail in a later blog.
Relative Density
The relative density, also referred to as specific gravity, is the ratio of the density of a substance to the density of a standard substance under specified conditions. When considering flammable vapours, it is often compared with the density of air (i.e. the relative density of air = 1).
In simple terms, if relative density of a flammable vapour is greater than 1 then it is heavier than air; if it is less than 1 then it is lighter.
This is particularly important when considering potential leaks of a flammable substance outside of normal containment. Whether or not a flammable vapour will settle at low or high levels will inform how you will design your safety measures such as local extract ventilation (LEV).

In the next blog, we will be looking at characteristics which apply to flammable solids, such as combustible dusts and powders.
If you require further information or assistance please contact us via email at inco@occupli.com, by phone at 021 497 8100 or start an instant chat with us via the chat box in the bottom right-hand corner of your screen.
